For clinical cell therapy applications, there is a shift in the industry to cell media platforms that exclude serum and other animal-derived raw material components to establish more defined growth parameters to improve consistency, mitigate contamination and immunogenic risk, and reduce the cost of goods sold (COGS).
Conventional media used for isolation and expansion often include supplementation with fetal bovine serum (FBS) at 5–20% (v/v). FBS has been a widely utilized media additive because it contains a high level of nutritional and physiochemical compounds required for attachment and growth.
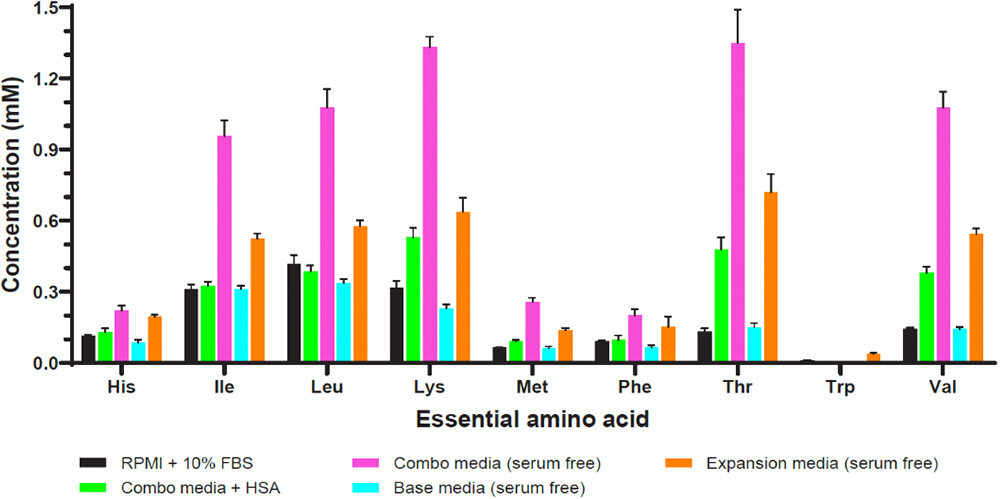
However, safety and regulatory concerns raised by the use of animal serum for clinical use of autologous or allogeneic human blood-derived materials has increased interest in identifying defined serum-free media formulations. Identifying critical factors and their concentrations with an eye towards designing an ideally formulated, defined serum-free medium should be carried out using rational and systematic approaches. In-depth global analysis of serum-containing media can facilitate reformulate efforts to replace serum with more defined components or to provide insight into commercially available, proprietary formulations (Figure 2)9,10.
The REBEL has a sensitive dynamic range of 5-100uM of detection to detect low levels of amino acids and other nutrients accurately, without interference from serum proteins, unlike other quantitative methods.
Implementing defined media formulations into cell therapy manufacturing would enhance consistency across cell bioprocessing protocols with the provision of a more uniform and controllable environment, which may be crucial to boost clinical efficacy.
Cell Culture Media Development and Optimization
In an autologous setting, each patient’s health status and demographics translates to a unique cell population, which imparts variability to the subsequent cell therapy manufacturing process emphasizing the importance of in-process controls to ensure a consistent drug product is produced in each and every manufacturing run even in the presence of such variability.
Process analytical technologies (PATs) can provide visibility into and information on critical quality attributes (CQAs) throughout the manufacturing process and QC release testing where speed, accuracy and throughput are needed to provide real-time process feedback for optimization efforts and to speed product release. The focus of PAT within the Quality by Design (QbD) framework has highlighted a need for better analytical tools that can allow rapid and frequent monitoring to inform process development in real-time. Current analytical methods suffer from long lag time between sampling and data generation ranging from days to weeks, which is prohibitive to effective design of experiment (DOE) methodology. Additionally, these assays may lack the resolution needed to show subtle changes that may result from incremental process optimization steps.
References:
- Medvec AR, Ecker C, Kong H, et al. Improved Expansion and In Vivo Function of Patient T Cells by a Serum-free Medium. Mol Ther Methods Clin Dev. 2017;8:65-74. Published 2017 Nov 7. doi:10.1016/j.omtm.2017.11.001
- Jung S, Panchalingam KM, Rosenberg L, Behie LA. Ex vivo expansion of human mesenchymal stem cells in defined serum-free media. Stem Cells Int. 2012;2012:123030. doi:10.1155/2012/123030