
Gene Therapy
Optimized Viral Vector Production
Gain insights and optimize viral vector manufacturing with actionable information on critical process parameters. Improve viral vector titer and accelerate gene therapy development with accessible PAT solutions.
Understanding Gene Therapy Manufacturing Complexities
Viral Vectors in Gene Therapy Manufacturing
Gene therapy is a rapidly developing class of biotherapeutics used to replace, insert, or delete genetic material in patients’ cells to treat or cure previously untreatable diseases, such as inherited retinal disease, spinal muscular atrophy (SMA), and sickle cell anemia. While there are several methods of delivering genetic material to cells, the use of viral vectors as the delivery system in vivo is the most widely adopted in the industry. The choice of the vector depends on the efficiency of transgene expression, ease of production, and safety. Common viruses used include Adeno Virus (AV), Adeno-Associated Virus (AAV), Lentivirus (LV), and Retrovirus (RV).
Generation of Genetic Material
Manufacturing of viral vectors is a complex process involving several steps. Typically, it begins with the generation of genetic material to be used for producing the viral vectors. The material used depends on vector type and may include plasmids and helper transducing viruses. Plasmids (pDNA) are manufactured via fermentation of genetically engineered bacteria, such as the Escherichia coli (E. coli). While this process is generally straightforward, without proper optimization, the plasmid DNA yield, topology, and stability may not be suitable for efficient commercial-scale GMP manufacturing. Microbial cultures require specialized growth medium as well as careful monitoring and control of critical process parameters (CPPs) during fermentation.
Host Cell Transfection and Vector Generation
The next step involves production of the viral vector in host cells using one of three methods: stable packaging cell lines, helper virus infection, or transient transfection. Although insect cells, such as Sf9 and other cell types, are sometimes used in production of viral vectors, Human Embryonic Kidney 293 (HEK293) cell lines are the most used to produce viral vectors. HEK293 cells have high transfection efficiency, can produce a variety of different viral vectors (Adeno-associated viruses and Lentiviruses for example) and can be adapted to suspension culture in serum-free medium. This achieves high cell densities and provides suitable glycosylation patterns for efficient virus replication.
Common Challenges in Upstream Viral Vector Manufacturing
There are several challenges related to upstream viral vector manufacturing process (host cell expansion, transfection, and viral vector generation) that need to be addressed to reduce cost, improve safety and efficacy of viral vector-based gene therapies:
- Adapting cells from adherent to suspension culturing and process scale up
- Maintaining high cell density and cell viability while minimizing aggregation during cell culture
- Optimizing transfection protocols to achieve high transfection efficiency
- Achieving high viral titer
- Obtain high ratio of full vs empty viral vector capsids
Optimizing viral vector production is complicated by the changes in metabolic requirements of host cells over the course of the cell culture, for example after cells are transfected. Furthermore, viral replication induces significant changes in the physiology and metabolic state of cells, impacting virus synthesis, assembly, and release. Understanding and modulating cellular metabolism through media development and dynamic control of cell culture parameters can help manufacturers increase viral vector titer, improve capsid quality and potency of gene therapies.
Viral Vector Media Matters
The culture medium and the feeding strategy must be developed and optimized to provide host cells with the essential nutrients to facilitate robust cell expansion, efficient transfection, achieve high viral titer, and support process scaleup. Different cell line lineages have varying gene expression profiles and often exhibit different metabolic needs after transfection and adaptation to suspension culture, hence media development should be tailored to each specific cell line, virus type, and culture process. While many commercial media formulations are available on the market, their composition and relative concentrations of critical components can vary greatly from vendor to vendor and from lot to lot. Furthermore, transfection media may be optimized for high growth, but not for high viral titer, therefore significant production media optimization is often required. Understanding cell metabolism and the impact of medium components, such as amino acids and glucose, on viral titer is key to increasing productivity.
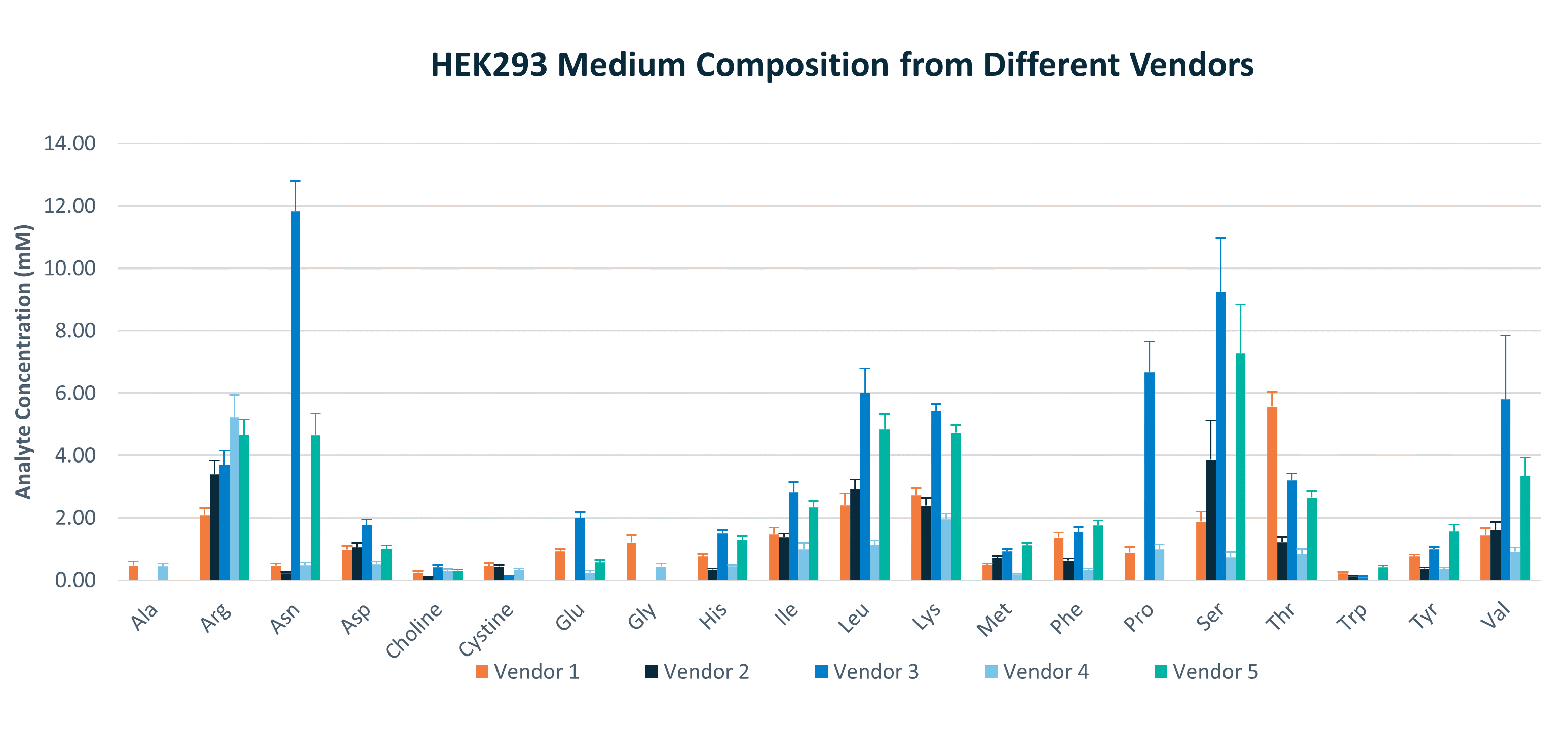
Learn What Nutrients Cells Need for Optimal Expansion & Viral Vector Production
REBEL provides a tool to support medium screening and optimization. HEK293 media from five different vendors show high diversity in nutrient composition. When starting your development work, do you know if you are screening a diverse panel, and how do you know if these media contain critical components for your process? For example, in a study by Nadeau et al. the role of asparagine (Asn) was noted as crucial for adenovirus virion formation in HEK2931. In the panel of media tested here Asn varies considerably between the five media analyzed with REBEL.
With REBEL, you will gain actionable insights into composition of media in the panel – and that can explain a lot of the outcome.
[1] Nadeau I, Jacob D, Perrier M, Kamen A. 293SF metabolic flux analysis during cell growth and infection with an adenoviral vector. Biotechnol Prog 2000;16:872–4

Accelerate Media and Feed Development for Faster Time to Market
Developing the optimal media formulation and feeding strategy necessitates frequent monitoring of key nutrients’ concentration through the cell expansion, transfection and viral vector production.
Waiting weeks for cell culture media analysis results is not feasible. REBEL provides a solution with its rapid analysis turnaround time, small sample volumes, and minimal sample prep.
Application Spotlight
Optimization of Scalable rAAV Production for Gene Therapy
We’re collaborating with MIT researchers in their quest to develop a scalable and efficient AAV vector production process. This research centers on the AAV produced through the triple-plasmid transfection method in HEK 293 cells, which is a widely utilized viral vector (VV) due to its non-pathogenic nature, broad tropism, ability to engineer its properties, and established manufacturing platforms. Despite the method’s established status, challenges persist in achieving a high VV titer and a favorable ratio of full to empty capsids.
Learn more in the webinar on demand or the poster from Advanced Manufacturing Cell and Gene Therapies Conference about the approaches to address these technological gaps including:
- Use of model-driven approach to design, predict, test, and optimize VV production processes.
- Benefit of using high-throughput metabolite screening to optimize AAV production using triple transfection method.
- Scale up and transfer to a continuous process at a bioreactor scale.
Screen and Optimize Your Cell Culture Media At-Line
Ensure Process Robustness with Real-Time Monitoring
Real-time monitoring and control of physicochemical variables such as pH, dissolved oxygen, and temperature is well established in cell culture processes but is not sufficient for developing and maintaining an optimized viral vector manufacturing process. Regardless of the bioreactor type or the mode of operation (fed-batch or perfusion), continuous monitoring and real-time process control can help achieve several important objectives in gene therapy manufacturing such as increasing viable cell density, improving viral titer, optimizing transfection, reducing media usage, and reducing toxic by-products.
Real-time monitoring of critical process parameters (CPPs) during cell expansion, transfection and viral vector production enhance process understanding, support development of more robust process models, and enable development and implementation of optimized substrate feeding and media exchange strategies.
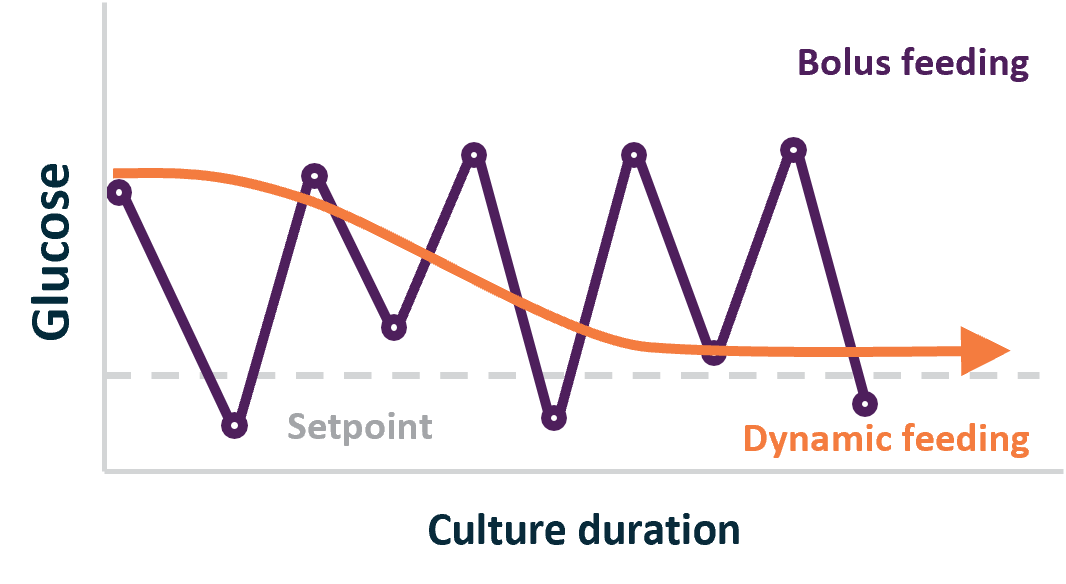
Glucose, being the primary energy source for cells, must be maintained at optimal concentration throughout cell culture duration to provide necessary nutrients and avoid cell stress. Cells’ nutrient requirements do not stay constant through the phases of culture and viral vector production. Nutrient requirements vary with the phase of cell growth, affected by transfection and viral vector generation. Without tight glucose control, enabled by continuous monitoring, glucose levels will fluctuate and destabilize cellular metabolism leading to cell stress and negative effect on viral vector production. Suboptimal glucose levels may lead to variations in vector characteristics such as infectivity, transgene expression levels, and vector stability. Tight control over glucose concentration allows for consistent production of high-quality viral vectors with reproducible properties. Real-time monitoring of glucose helps implement dynamic control of feeding and media exchange which can significantly reduce media and feed usage by as much as 60% without affecting productivity2.

In addition to glucose, lactate is another critical parameter that needs to be continuously monitored. Lactate accumulation can indicate metabolic stress in the cells, which can compromise their ability to produce viral vectors. Additionally, lactate accumulation can lead to decrease of media pH and negatively affect cell growth, viability, as well as the stability of viral vectors produced.

Real-time total biomass measurements, correlated to the total cells counts, is vital for ensuring consistency and efficiency of viral vector manufacturing. Total biomass information can help identify deviations from the expected cell growth trajectories and maintain process parameters within the specified operating ranges. Transfection efficiency, the proportion of cells that successfully uptake the plasmid DNA, is influenced by cell density hence, real-time total cell count information can be used to optimize transfection protocols.
The success of the triple transfection process relies on optimizing key parameters of cell culture and refining the feeding strategy. Critical parameters, such as glucose and lactate, impact cell viability, transfection efficiency, viral vector titer and quality, hence, must be meticulously monitored and controlled during the cell culture process.
[2] Valkama AJ, Leinonen HM, Lipponen EM, Turkki V, Malinen J, Heikura T, Ylä-Herttuala S, Lesch HP. Optimization of lentiviral vector production for scale-up in fixed-bed bioreactor. Gene Therapy. 2018 Jan;25
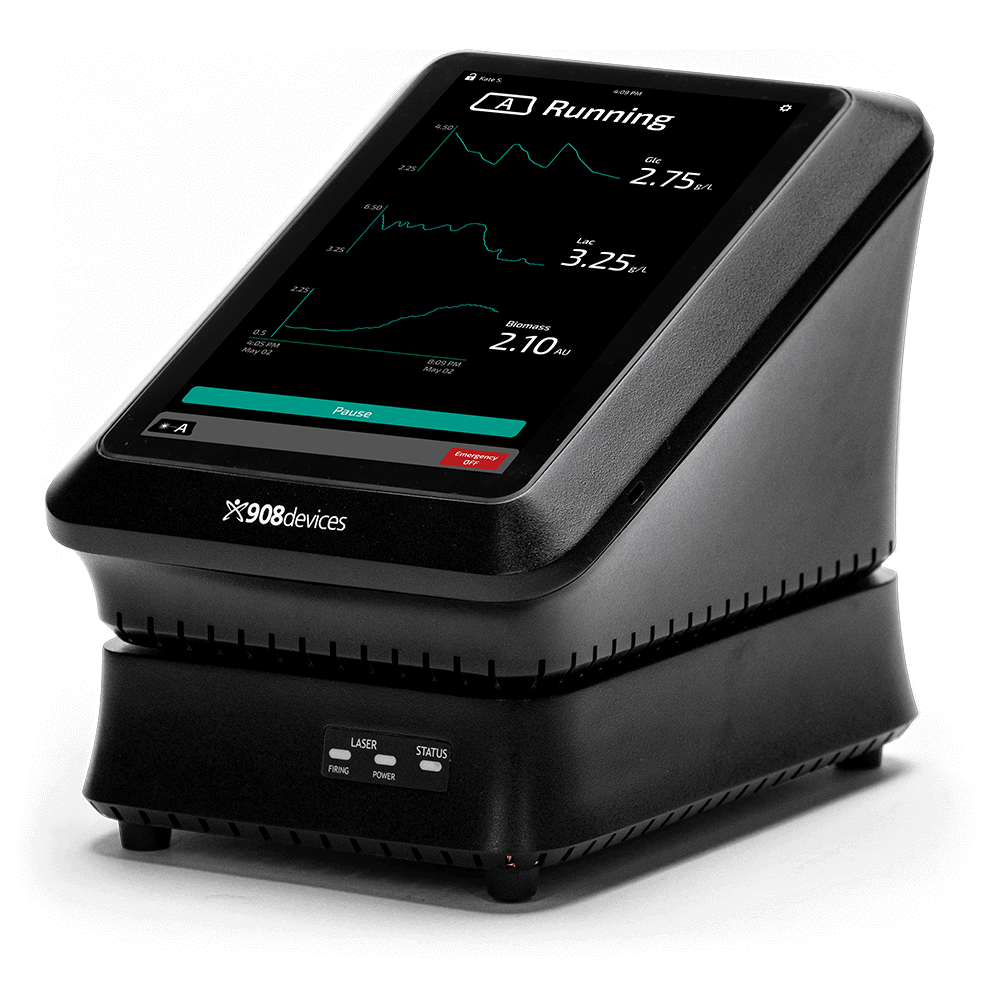
Instant PAT Implementation with Raman-based MAVERICK
This application note describes how MAVERICK, a Raman spectroscopy-based PAT device, was used to monitor critical process parameters without the need for chemometric calibration modeling. Learn about results from experiments that show how real-time monitoring is essential to maintaining optimal cell culture conditions, ensuring product quality, and maximizing yield.

At-line Amino Acids Monitoring
Rapidly screen commercial media and develop optimal media and feeding strategies to increase titers and quality of viral vectors with diverse cell lines.
Real-time Glucose Monitoring
Dynamically control glucose feeding to optimize cell expansion, reduce media usage, and maintain consistent infectivity, transgene expression levels, and vector stability.
Real-time Lactate Monitoring
Detect and mitigate process deviations and prevent toxic levels of metabolites that affect cell health, proliferation, and quality of produced viral vectors.
In-line Biomass Measurement
Optimize transfection protocols, monitor cell proliferation, and control perfusion processes.
Your Choice for Ultimate Monitoring and Control of Viral Vector Production
Our suite of devices provides the data you need, where and when you need it.
Implement Process Analytical Technology (PAT) into Your Gene Therapy Workflows
To reduce costs, improve manufacturing efficiencies and increase patient accessibility to life-saving treatments, gene therapy developers need an array of tools that enable them to monitor and control their process in real time. MAVERICK and MAVEN are PAT solutions that can be easily integrated with a number of manufacturing automation systems, offering real-time process control.
For Cell Culture Media Analysis
REBEL
Analyze fresh or spent media and get quantitative reports for amino acids, dipeptides, water soluble vitamins and biogenic amines.

For Glucose & Lactate
MAVEN
Get valuable insights and control from real-time glucose and lactate monitoring without the loss of bioreactor or fermenter volume.

For Bioprocessing
MAVERICK
Raman-based, in-line monitoring and control of multiple bioprocessing key process parameters in up to six bioreactors simultaneously.
Suggested Resources
Screening of chemically-defined media for suspension cultures of HEK293 cells
Across four vendors, there was a high variation in the fresh media composition.
On-line Measurement of Glucose and Lactate with Automated Feedback Control
MAVEN answers the urgent need for on-line measurements and automated control of glucose levels, which reduces manual labor, lowers risk of contamination from sampling, decreases risk of human error with feeding, and reduces the stress that cells may experience from variable glucose levels.
Instant Implementation of Raman-based PAT with MAVERICK for Monitoring Glucose and Lactate
MAVERICK is easy to set up and enables instant implementation of PAT generating accurate, precise, linear and selective in-line measurements across a wide range of media used typically in mammalian (including CHO and HEK293 media) cell culture for the production of biotherapeutics.
REBEL Brochure
The REBEL is a novel, dedicated cell culture media analyzer designed for rapid, easy, and efficient amino acid and nutrient analysis.
MAVEN Brochure
MAVEN offers monitoring and control of glucose and lactate concentrations during cell culture and fermentation. Download this brochure to learn more.
MAVERICK Brochure
Real-time bioprocess monitoring and control, right out of the box. In-line measurements of multiple critical parameters across media, across processes, across mammalian cell lines, across scales.
Subscribe to Our Communications
Signup to receive new product updates, technical tips and more.
