Cell Culture Media Development >> Gene Therapy Process Development
Plasmid DNA Productivity & Cell Culture Media Analysis
As more cell and gene therapies advance to FDA approval, it is essential to scale up current production processes to increase plasmid DNA productivity
Read the other articles in this series:
Cell Culture Media Analysis for Gene Therapy Process Development
Viral Vector Manufacturing & Cell Culture Media Analysis
Cell Culture Media Impact on Viral Vector Scalability
Cell Culture Media Impact on Viral Vector Safety
As more cell and gene therapies advance to FDA approval, it is essential to scale up current production processes to increase plasmid DNA productivity economically, and at the scale needed to meet industry demand.
Plasmid production is commonly achieved through recombinant Escherichia coli fed batch fermentation, during which the appropriate genetic sequences are amplified, harvested, purified, and tested for safety. There can be issues with lot-to-lot consistency in the fermentation media which contains hydrolysates, which in turn imparts lot-to-lot variability in plasmid yield and purity. In addition to this, the industry has the desire to move to GMP-compliant plasmid manufacturing. Plasmid DNA production provides the upstream raw material for viral vector production for clinical applications. These have an increasing level of stringent requirements and call for GMP plasmid manufacturing with more defined culture media/growth conditions without animal-derived components, better in-process monitoring, and overall process control 1.
Typical fermentation media is composed of undefined components, like peptone or hydrolysate mixtures with a wide range of amino acid concentrations (Figure 1) resulting in yield, quality and purity inconsistencies in manufacturing. Here, the REBEL analyzer can be leveraged to check media conformity across different lots of fermentation media and between vendors to mitigate inconsistencies during manufacturing.
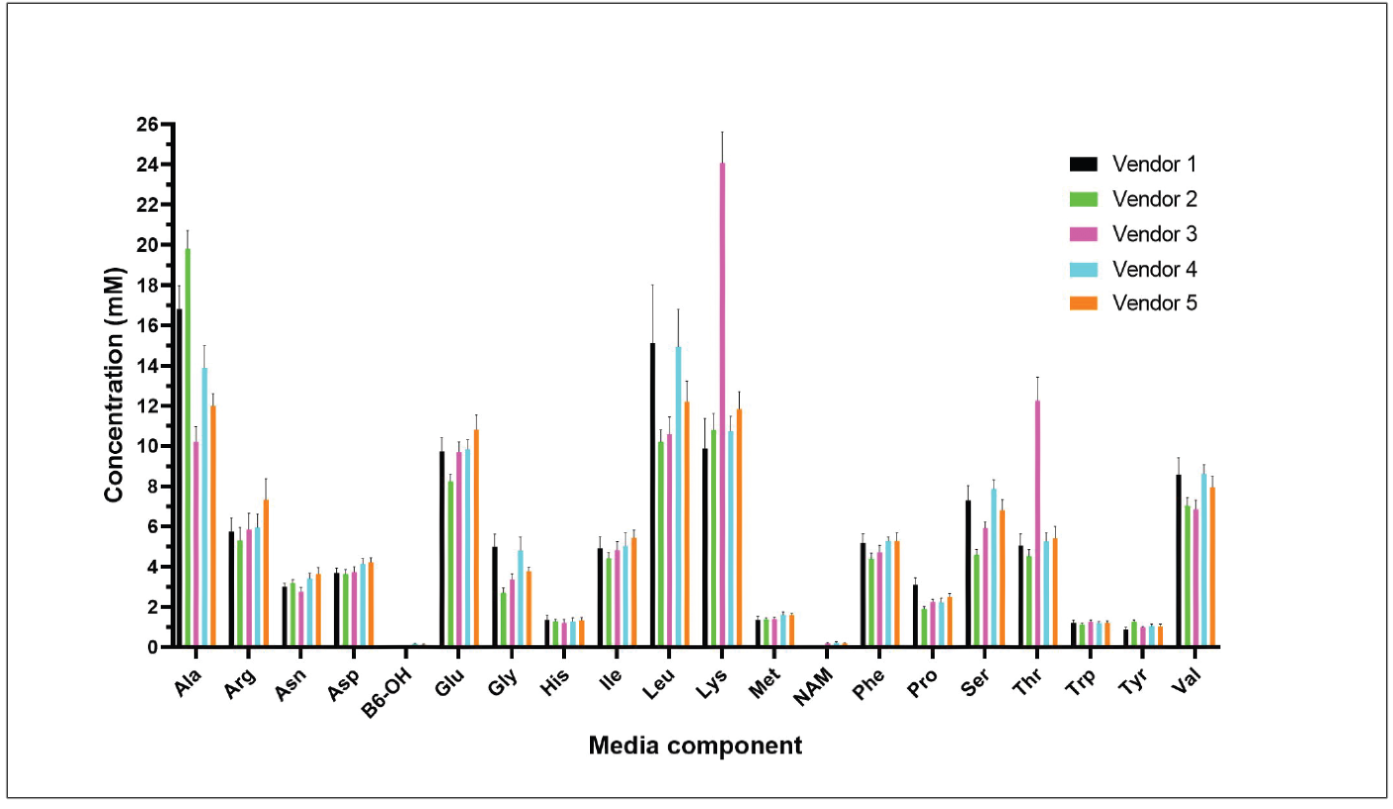
Additionally, in-depth media analysis can aid in the movement towards chemically defined fermentation media to meet increased regulatory standards and to achieve optimal productivity and plasmid quality necessary for downstream viral vector production.
References
1. Hitchcock T. Manufacturing Plasmid DNA: Ensuring Adequate Supplies for Gene and Cell Therapies. Bioprocess International. October 17, 2016. https://bioprocessintl.com/manufacturing/cell-therapies/
manufacturing-plasmid-dna-ensuring-adequate-supplies-gene-cell-therapies/.

On-Demand Webinar
The Critical Role of Cell Culture Media Analysis in CGT Development
Does cell culture analysis matter for CGT development? This short webinar explores what is already known, reviews at-line methods for cell culture media analysis, and shares recent data from two case studies.
Keep Reading About CGT Process Development

Download the E-Book
What’s In Your Media?
Take a deep dive into the role that cell culture media analysis plays across bioproduction, cell therapies, and gene therapies.

Easy to understand answers
No more waiting for third party testing
Accurate, reliable performance whether running 1 or 100 samples
Solution for Cell Therapy Process Development
The REBEL
At-line cell culture media analysis. Results on 30+ components in under 10 minutes. When and where is your call. With the REBEL, now is always on the table.
email Subscribe to Our Communications Signup to receive new product updates, technical tips and more.