Cell Culture Media Analysis>> Gene Therapy Process Development
Cell Culture Media Impact on Viral Vector Quality & Safety
Viral vector quality requires serum-free production using more defined, animal-free and/or chemically-defined medium
Read the other articles in this series:
Cell Culture Media Analysis for Gene Therapy Process Development
Productivity for Plasmid DNA Production & Cell Culture Media Analysis
Viral Vector Manufacturing & Cell Culture Media Analysis
Cell Culture Media Impact on Viral Vector Scalability
Cell Culture Media Impact on Viral Vector Safety
As with cell therapy manufacturing, viral vector serum-free production aims at more defined, animal-free and/or chemically-defined medium that mitigates any potential risk of transfer of viruses or prions into the final product that could induce an undesirable immunogenic reaction in the patient.
In addition, it is important to consider whether the vector bulk can be sterilized. For instance, viral clearance is used to some degree in both AAV and Lentivirus processes, which confers a level of viral safety.1 Furthermore, as viral vector demand climbs with more products reaching late-stage large-scale manufacture, supply limitations of certified serum are a real possibility1. In turn, the reduced reliance on serum with serum-free media for adherent HEK293 cells, the improved lot-to-lot consistency, and the need for more defined mammalian cell growth conditions have driven the development of chemically defined media formulations that are both serum-free and protein-free.
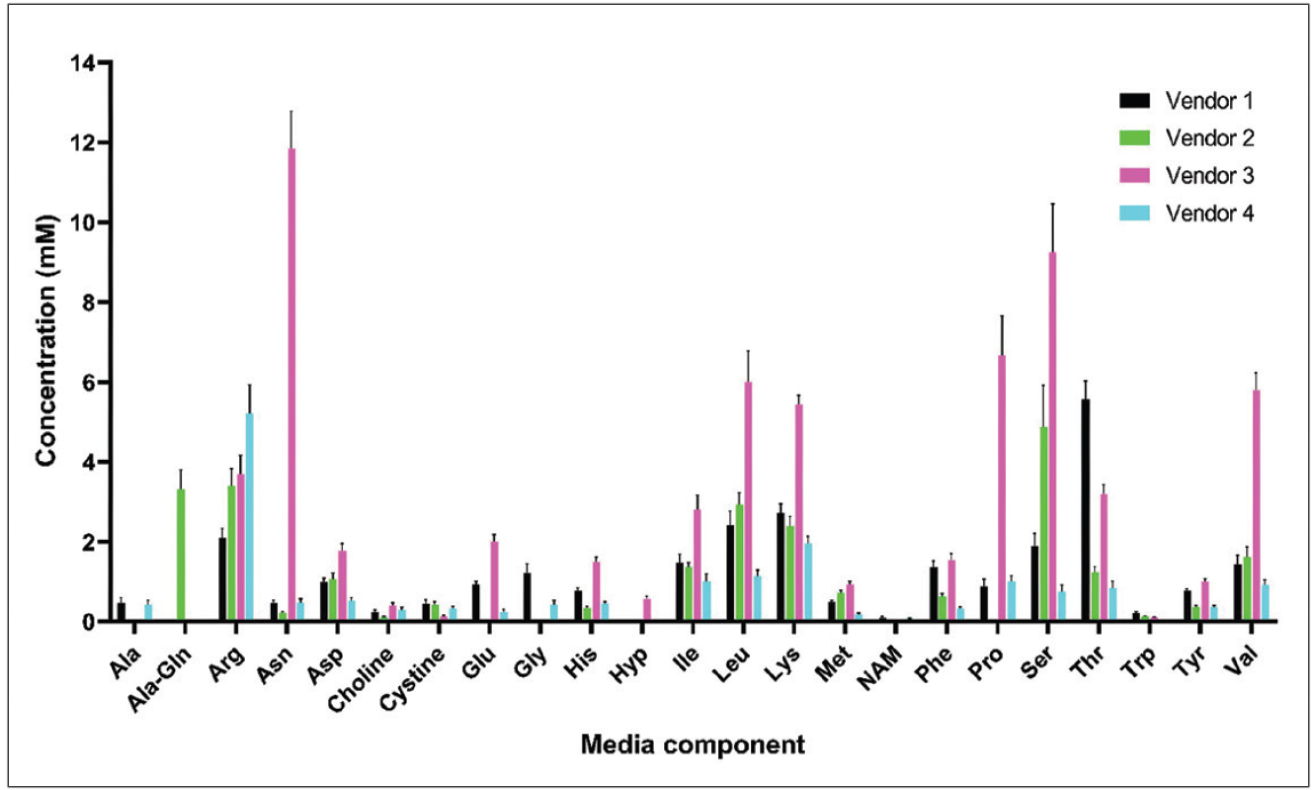
Commercially sourced chemically defined formulations are available but many of these formulations are proprietary and thus pose hurdles in optimization for a specific vector product because the components and their concentrations are unknown2, which is where the REBEL can provide valuable intel and insight to drive optimization (Figure 1).
As in monoclonal antibodies where the cell culture medium can also impact PTM and other CQAs, the medium also could have an impact on the capsid PTM and associated CQAs as capsid proteins are glycosylated3. These are direct indicators of viral vector quality require optimization of the cell culture media used. Also medium optimization needs to be taken into consideration during scale up as cell nutrient requirements can change as the scale of the bioreactor increases.
When developing a new AAV or LV process, decisions have to be made on cell line, mode of culture, and which cell culture medium and feeding strategy to use for optimal productivity and desired CQAs. One common strategy is to perform a screening of commercially available cell culture media. For example there are multiple commercial media available for HEK293 cells, one of the most used cell lines in viral vector production. As shown in Figure 1, these media can have similar or widely different compositions in terms of amino acids. Is it beneficial to screen as diverse set of medium as possible to maximize the chance to identify a medium that provide the right nutrients to a specific HEK293 cell line. There are many sub-types of HEK293 also available and each one may need a different balance of AA and vitamins.
References
- Petit S, Glover C, Hitchcock T, Legmann R, Startt D. Upstream Manufacturing of Gene Therapy Viral Vectors. Cell Culture Dish. January 20, 2021. https://cellculturedish.com/upstream-manufacturing-gene-therapy-viral-vectors-2/.
- Gélinas JF, Davies LA, Gill DR, Hyde SC. Assessment of selected media supplements to improve F/HN lentiviral
vector production yields [published correction appears in Sci Rep. 2018 Apr 25;8(1):6774]. Sci Rep. 2017;7(1):10198. Published 2017 Aug 31. doi:10.1038/s41598-017-07893-3 - Aloor A, Zhang J, Gashash EA, Parameswaran A, Chrzanowski M, Ma C, Diao Y, Wang PG, Xiao W. Site-Specific
N-Glycosylation on the AAV8 Capsid Protein. Viruses. 2018 Nov 17;10(11):644. doi: 10.3390/v10110644. PMID: 30453606; PMCID: PMC6266768.

On-Demand Webinar
The Critical Role of Cell Culture Media Analysis in CGT Development
Does cell culture analysis matter for CGT development? This short webinar explores what is already known, reviews at-line methods for cell culture media analysis, and shares recent data from two case studies.
Keep Reading About CGT Process Development

Download the E-Book
What’s In Your Media?
Take a deep dive into the role that cell culture media analysis plays across bioproduction, cell therapies, and gene therapies.

Easy to understand answers
No more waiting for third party testing
Accurate, reliable performance whether running 1 or 100 samples
Solution for Cell Therapy Process Development
The REBEL
At-line cell culture media analysis. Results on 30+ components in under 10 minutes. When and where is your call. With the REBEL, now is always on the table.
email Subscribe to Our Communications Signup to receive new product updates, technical tips and more.