Cell Culture Media Analysis>> Gene Therapy Process Development
Cell Culture Media Impact on Viral Vector Production Scalability
To meet market demands in gene therapies, improvements must be made to viral vector production scale-up.
Read the other articles in this series:
Challenges in Gene Therapy Process Development
Productivity for Plasmid DNA Production & Cell Culture Media Analysis
Viral Vector Manufacturing & Cell Culture Media Analysis
Cell Culture Media Impact on Viral Vector Safety
In order to meet market demands in gene therapies, there is a need to improve production efficiencies, which will reduce manufacturing costs and improve patient access.
As an example, a phase 1/2 trial for hemophilia B required over 400 ten-layer cell stacks to generate sufficient material for six patients1. While the trial was a success, this study highlights the need for new methods to improve vector generation especially if gene therapies are adopted for larger disease indications. The industry is investigating improving viral vector productivity on multiple levels, AAV and lentivirus titer, overall particle quality as well as the inclusion of the genes of interest in the virus particles.
The ability to culture the production cells at higher densities than is possible in adherent monolayers is one way to increase titers. Viral vector production scale-up starts with adapting adherent HEK293 cells to grow in suspension in a bioreactor system. This provides a more robust and scalable process enabling higher cell densities to increase productivity as well as reducing overall labor and materials costs2. However, adapting a cell line to grow in suspension requires medium and process development time that the industry can ill-afford. As an alternative, microcarrier technology for adherent cell lines has also been considered for scale up. This offers advantages of providing a larger surface area for cell growth and the ability to utilize bioreactor platforms that are more suited for large-scale production without the need to adapt the cells to non-adherent conditions3. In stark contrast to conventional bench-scale static cultures, large-scale suspension bioreactor cultures impart complex hydrodynamic forces on cells due to the fluid agitation required to aid in the transport of nutrients and gasses within the culture volume.5,6 The environmental perturbations and increased cell densities that the cells experience in bioreactor environments may alter their nutrient requirements or require additional additives to serve as a protectant against shear stress6 (i.e., Pluronic F68, methylcellulose or dextran) and thus may require the optimization of the cell culture media in process development.
The use of any animal derived product in medium, including serum, increases the risk of contamination, supply chain instability and variability. However, removing serum and optimizing media for production of viral vectors does require extensive media development and additional time and resource commitment. HEK 293 has been used extensively for transient protein expression, and thus, commercially available chemically defined media for HEK293 may not be optimized for production of viral vectors. Viral vector production is more complex than single protein production, such as monoclonal antibodies. In addition, transfection media may be optimized for high growth, but not for producing high viral titers, therefore significant optimization is required to meet the specific needs of viral vector production culture. Understanding cell metabolism and the impact of medium components, like amino acids and glucose, on titer levels can be done using media analysis studies and is key to increasing productivity.
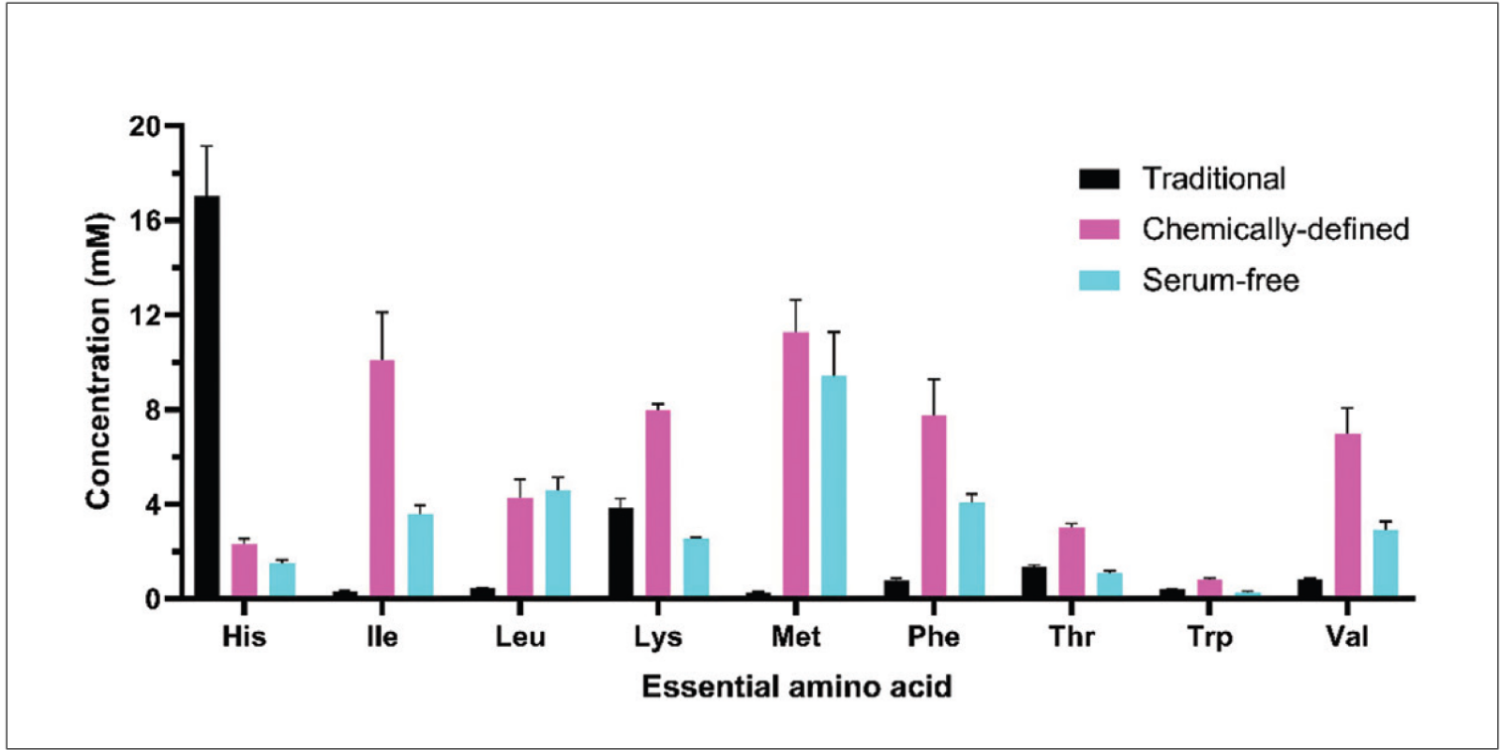
Additionally, the FDA has expressed concern over the use of HEK293T cells owing to the inclusion of the SV40 large T antigen DNA sequence. This oncogenic construct could pose the risk of tumor development in patients, which has prompted manufacturers to investigate other HEK293 clones and other cell platforms. The baculovirus- Spodoptera frugiperda insect cell (Sf9) platform is an attractive alternative to mammalian HEK293T cells and holds potential as a GMP-compatible viral production system.7 In implementing insect cell lines, the selection of the appropriate media, in particular, the amino acid content, is important because many amino acids required for their growth and/or protein production are not synthesized by insect cells, and must be supplied by the culture media. As shown in Figure 1, the amino acid profile across several commercial formulations of insect cell media varies across a wide range. This global analysis of amino acid content coupled with an understanding of insect cell metabolism can inform media optimization efforts during process development to modulate critical parameters like cell growth/viability and productivity.
References
- Hitchcock T. Manufacturing Plasmid DNA: Ensuring Adequate Supplies for Gene and Cell Therapies. Bioprocess International. October 17, 2016. https://bioprocessintl.com/manufacturing/cell-therapies/manufacturing-plasmid-dna-ensuring-adequate-supplies-gene-cell-therapies/
- Mirasol F. Modernizing Bioprocessing for Gene Therapy Viral Vectors. Pharmaceutical Technology 2020;44(10):28–33
- Zhao H, Lee KJ, Daris M, et al. Creation of a High-Yield AAV Vector Production Platform in Suspension Cells Using a Design-of-Experiment Approach. Mol Ther Methods Clin Dev. 2020;18:312-320. Published 2020 Jun 3. doi:10.1016/j.omtm.2020.06.004
- Yang J, Guertin P, Jia G, Lv Z, Yang H, Ju D. Large-scale microcarrier culture of HEK293T cells and Vero cells in single-use bioreactors. AMB Express. 2019;9(1):70. Published 2019 May 24. doi:10.1186/s13568-019-0794-5
- Weidner T, Druzinec D, Mühlmann M, Buchholz R, Czermak P. The components of shear stress affecting insect cells used with the baculovirus expression vector system. Z Naturforsch C J Biosci. 2017;72(9-10):429-439. doi:10.1515/znc-2017-0066
- Berry JD, Liovic P, Šutalo ID, Stewart RL, Glattauer V, Meagher L. Characterisation of stresses on microcarriers in a stirred bioreactor. Applied Mathematical Modelling. 2016;40(15-16): 6787-6804 doi: 10.1016/j.apm.2016.02.025.
- Merten OW. Advances in cell culture: anchorage dependence. Philos Trans R Soc Lond B Biol Sci. 2015;370(1661):20140040. doi:10.1098/rstb.2014.0040

On-Demand Webinar
The Critical Role of Cell Culture Media Analysis in CGT Development
Does cell culture analysis matter for CGT development? This short webinar explores what is already known, reviews at-line methods for cell culture media analysis, and shares recent data from two case studies.
Keep Reading About CGT Process Development

Download the E-Book
What’s In Your Media?
Take a deep dive into the role that cell culture media analysis plays across bioproduction, cell therapies, and gene therapies.

Easy to understand answers
No more waiting for third party testing
Accurate, reliable performance whether running 1 or 100 samples
Solution for Cell Therapy Process Development
The REBEL
At-line cell culture media analysis. Results on 30+ components in under 10 minutes. When and where is your call. With the REBEL, now is always on the table.
email Subscribe to Our Communications Signup to receive new product updates, technical tips and more.