In-depth analysis of top selling biotherapeutics

H I G H L I G H T S
- Charge variant analysis of top selling monoclonal antibodies.
- Demonstrated ease of use, speed and depth of analysis obtained using the ZipChip® system, which couples microfluidic capillary zone electrophoresis (CE) separation of intact charge variants with direct electrospray ionization mass spectrometry (ESI-MS) analysis.
- The benefits of the ZipChip system include:
-Minimal sample preparation involved for many monoclonal antibody formulations.
-In the same fast analysis, a charge variant profile is obtained and is coupled with on-line MS identification in native conditions.

“Using the ZipChip system provides us with the ability to obtain charge variant separation in less than 15 minutes. This is a real advantage for our lab.„
- Sara Carillo
APPLICATION DEVELOPMENT TEAM LEADER, NIBRT
Materials & Methods
100 μg of each monoclonal antibody were prepared at a concentration of 0.5 mg/mL following the ZipChip protocol for intact antibody charge variant analysis.
| ZipChip Protocol | Boosting Sensitivity for Intact Antibody Charge Variant Analysis |
| Assay Kit | ZipChip Native Antibody Kit |
| Chip Type | ZipChip HRN |
| Field Strength | 500 V/cm |
| Run Time | 15 minutes |
| Mass Spec Type | Thermo Scientific QExactive™ Plus Hybrid Quadrupole Orbitrap with Biopharma Option |

Introduction
The biopharmaceutical industry continues to develop mAb-based biotherapeutics for several applications and disease treatments. The complexity of these molecules creates a considerable challenge for the analytical technologies used to monitor the product quality attributes (PQAs) that need to be measured and controlled to guarantee safety and efficacy1. According to ICH guidelines, one of the features that needs to be monitored during biopharmaceutical development, and during approval and batch release post authorization, is the charge variant profile of a biotherapeutic. Standardized methods include the use of capillary electrophoresis using either zone or isoelectric focusing separation modes or the use of ion exchange liquid chromatography with UV detection (IEX-UV)2. The recent development of native mass spectrometry has facilitated the use of such separation techniques with Orbitrap mass spectrometry detection to obtain on-line MS identification of the charge variant proteoforms3,4. Native MS offers several advantages that include fast analysis and minimal sample preparation when combined with an upfront analytical separation, thus avoiding artificially-induced modification on the analyte.
ZipChip is a fast and reproducible tool for qualitative and quantitative charge variant analysis.
We tested the performance of the ZipChip system with a QExactive™ Plus Hybrid Quadrupole Orbitrap mass spectrometer to perform native MS experiments on the intact charge variants separated by ZipChip. The drug products tested in this work are rituximab, bevacizumab and trastuzumab.

Craig Jakes, PhD student, prepares the ZipChip system to begin a charge variant separation run
Results
The deep structural characterization of biotherapeutics is widely reported as a key step in new drug development and quality control to assess product safety and potency. Charge variant analysis is one of the product quality attributes that needs to be monitored during development and lot release to assess process robustness. In this study the ZipChip microfluidic capillary electrophoresis platform was applied for charge variant profiling of three top-selling drug products: rituximab, bevacizumab and trastuzumab.
For all the samples charge variant separation was obtained with little sample preparation and using a unique method for both CE separation and MS data acquisition. Moreover, the separation was obtained in less than 15 minutes in all the cases, providing a fast and reproducible tool for qualitative and quantitative charge variant analysis.
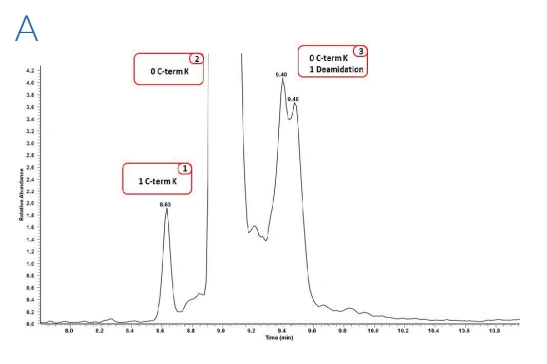
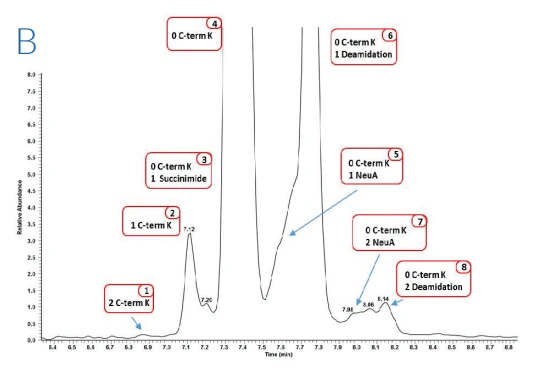
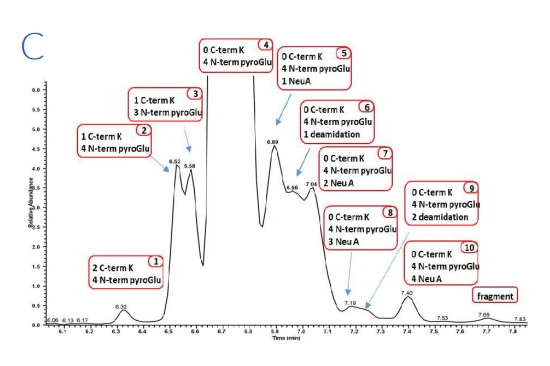
Figure 1: Zoom of the electropherograms obtained after ZipChip CE-ESI-MS analysis on bevacizumab (A), trastuzumab (B) and rituximab (C) drug products
Figure 1 shows the electropherograms obtained from the three drug products. For each molecule at least 10 proteoforms were identified, all presenting glycoform heterogeneity, with a total of up to 52 proteoforms identified for trastuzumab drug product. The use of QExactive™ plus mass spectrometer enables the fast acquisition of MS data using resolution setting of 35,000 at 200 m/z, routinely providing experimental masses with mass accuracies mostly below 20 ppm.
Due to the separation of the charge variants during the ZipChip separation, in addition to information on the glycoforms and C terminal lysine clipping, it was possible to determine pyro-glutamate formation and the presence of succinimide containing variants as well as deamidation
levels. These post-translational modifications are usually difficult to monitor due to the molecular mass of these variants being near isobaric to the main variant.
Overall, modifications with less than 0.1% abundancy have been confidently identified concluding that the ZipChip system is a powerful tool for biotherapeutics analysis.
Authors:
Sara Carillo, Craig Jakes, Jonathan Bones
National Institute for Bioprocessing Research & Training, Dublin, Ireland
References
1.Tassi M, De Vos J, Chatterjee S, Sobott F, Bones J, Eeltink S., J Sep Sci.,
2018; 41(1): 125-144. 2. Sosic Z, Houde D Blum A, Carlage T, Lyubarskaya Y,
Electrophoresis 2008; 29(21): 4368-4376. 3. Redman EA, Mellors JS, Starkey
JA, Ramsey JM. Anal Chem. 2016; 88(4): 2220-2226.
email Subscribe to Our Communications Signup to receive new product updates, technical tips and more.