Targeted metabolomics-based process analytical technology (PAT) allows for metabolic profiling of the T-cells throughout the manufacturing process through spent media analysis. Particularly with the adoption of more automated and closed processes for large-scale commercial manufacturing, analytical tools that can be operated at or on-line to minimize manual handling time are desirable. Traditionally, groups involved in cell therapy process development do not have access to the necessary analytical tools or resources required for this type of analysis. Outsourcing analysis to core labs or CROs can take days to weeks from sample-to-result, which can severely delay process development and PAT implementation efforts.
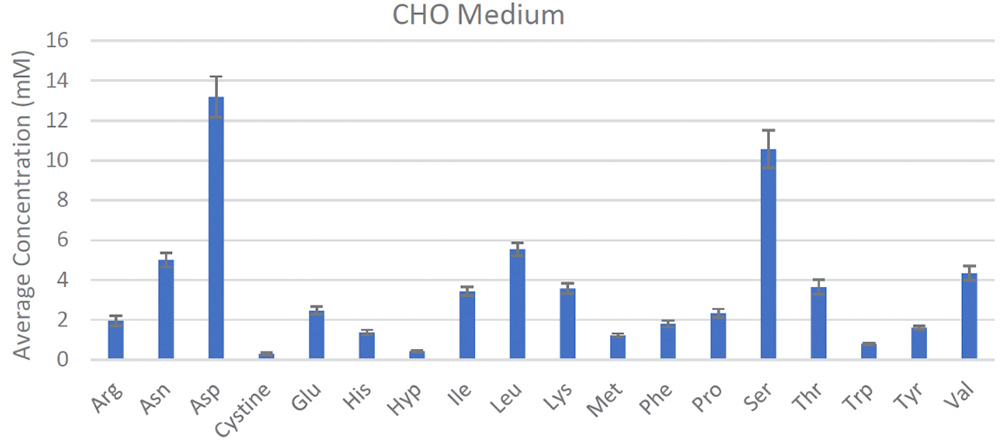
The REBEL is built for this purpose, enabling frequent, at-line measurements from the bioreactors for active, near real-time monitoring of CQAs, which has direct implications on the therapeutic efficacy and safety, to ensure product quality and batch-to-batch consistency. Its small, self-contained footprint fits alongside bioreactors and has the ability to rapidly analyze samples to provide quantitative identification and analysis on a panel of >30 analytes including all the key amino acids, biogenic amines, water soluble vitamins and dipeptides with accuracy and reproducibility (Figure 3). Integrating data reporting capabilities with portable report formats and automated performance qualification and 21 CFR Part 11-compliant software make the REBEL easy to integrate into cGLP/cGMP environments.
‘Scale out’ and ‘scale up’ are two paradigms to increase production for cellular therapies, each with their own distinct challenges. Scaling out involves increasing the number of operation units in parallel to increase capacity whereas, scaling up increases the size of the operation unit, which typically means moving to larger culture vessels that are different from what was used in small-scale production.
Scale-out options are easy to implement since the culture parameters do not change, but inefficiencies in the scale-out model may result in increased COGS that don’t meet long-term product needs. During scale up to larger bioreactor platforms, the change in vessel size, agitation and other physiological parameters can affect growth and cellular performance, which can require extensive process optimization to ensure that CQAs are still met. In some cases, it may be necessary to select a different cell culture media or the addition of additional factors to maintain phenotype control and optimize cell growth without inducing other changes that could result in a loss of functionality.
A Perspective On Outsourcing
In efforts to bring therapies to market sooner, many companies partner with CMOs and CDMOs to aid in the movement of their cell therapy products through all phases of clinical development. However, with regards to outsourcing, the developer may have less oversight over the manufacturing process, and disruptions at the CMO or CDMO can create bottlenecks for the developer, particularly if they rely on a single CMO or CDMO to fulfill their needs11.
Because many of the current generation of regenerative medicine products are unique and highly specialized, requiring customized components and non-standard technology approaches that need specially trained staff, traditional CMOs/CDMOs struggle to adapt to these bespoke requirements. This can ultimately delay tech transfer and extend timelines that negatively impact the developer and their patient population especially when speed to market is so important. Additionally, industry experts recommend that every developer should keep some aspect of manufacturing in-house even when partnering with a contract organization for certain aspects of the manufacturing process to build process knowledge and understanding, advantageous when seeking regulatory approvals12.
New analytical platforms like the REBEL have broad utility across cell therapies from development through to commercial manufacture. The speed and throughput of CE–MS based approaches to deliver in-depth spent and fresh media analysis can be leveraged to improve the process understanding and efficiency and maintain visibility and control throughout all stages of cell therapy production with the ultimate goal of bringing these life-saving therapies to patients faster.
References:
- Lehmicke M. Manufacturing Cures: Infrastructure Challenges Facing Cell And Gene Therapy Developers. Pharma Intelligence | Informa. June 10, 2019.
- Harris E. The Case For In-House Manufacturing Of Cell Therapies. Cell & Gene. August 18, 2020 https://www.cellandgene.com/doc/the-case-for-in-house-manufacturing-of-cell-therapies-0001.